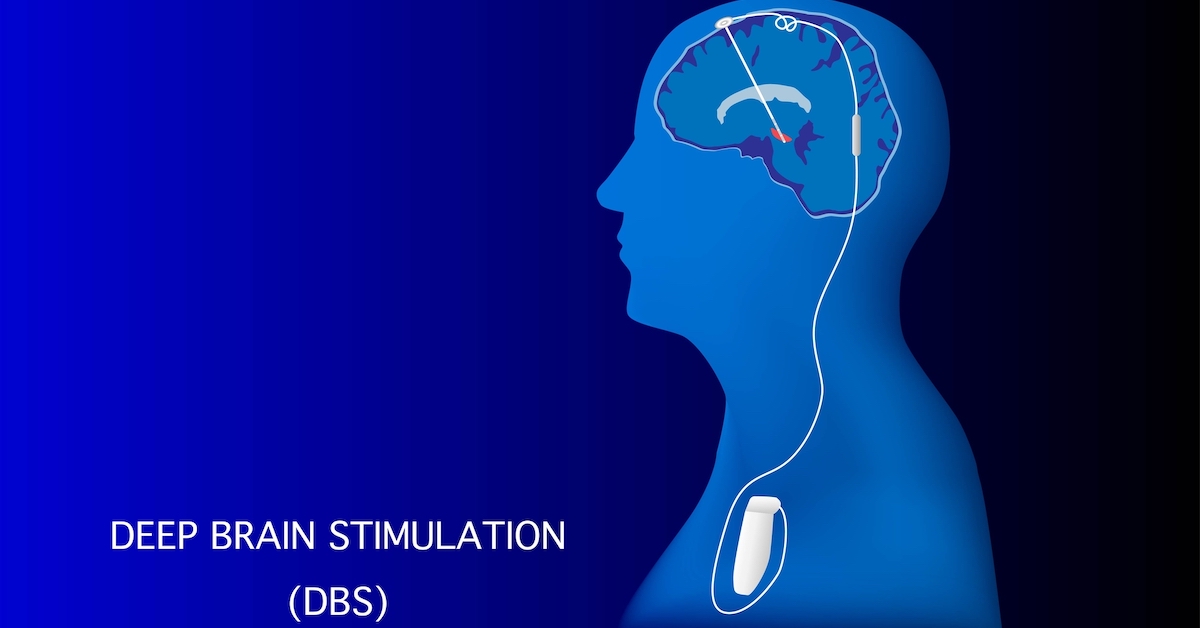
A hospital in England is the first in the world to implant a deep brain stimulation (DBS) device to deliver electrical impulses to areas of the brain impacted by Parkinson’s.
It’s the smallest DBS system ever created, and Tony Howells, the first surgical patient, is thrilled with the results saying, “It’s given me my life back.” He can now walk two miles instead of 200 yards and he’s back playing golf.
So, what does this mean for other Parkinson’s patients? Let’s take a closer look…
DBS is a technological breakthrough. It uses electrical impulses that target areas deep within the brain that are critical for regulating movement. While it can alleviate symptoms of Parkinson’s, it can’t stop the progression of the disease.
Current devices are like pacemakers, with the sizable battery having to be implanted in the chest while the leads are routed under the skin, up to the neck, and into the top of the skull. As you can imagine, it’s a complex and expensive procedure requiring two surgeries spaced three to six weeks apart with all the risks and complications that come with them.
The new device is different.
Quicker-Cheaper-Safer
The new system, called Picostim, is one third the size of current devices, allowing the electrodes and battery to be placed in the skull. This “brain pacemaker” allows surgery to be much simpler, taking three hours instead of six. It also eliminates the need for a third or fourth revision surgery necessitated by fractures to the lead.
What’s more, doctors say the new device reduces the risk of complications and infections, improves safety, has fewer side effects, a better cosmetic appearance and a lower cost compared to the conventional system.
Tony Howells is the first of 25 patients who are part of a trial scheduled to be completed next year.
Dr. Alan Whone, Consultant Neurologist at North Bristol NHS Trust, who is leading the trial, explained, saying, “We are delighted with how this first case went in the operating theatre and with how the patient’s symptoms have been improved over the last year. We are hopeful that if these findings hold-up, we will have a significant technical advance by which to improve Parkinson’s care across the world.”
One Happy Patient
A delighted Mr. Howells talked about his experience, saying, “I was first diagnosed with Parkinson’s nine years ago when I noticed a slight tremor in my right hand. The most difficult thing to accept is the decline in daily activities like tying-up shoelaces, taking three or four minutes instead of seconds.
“The surgery was quick and to my amazement when I woke-up I had no pain. I was operated on [on] a Wednesday and went home on Thursday. The impact has been amazing; the dystonia [muscle spasm] which is a side effect from the medication has gone. To say I am happy about having DBS is an understatement.”
While his review is glowing, not everyone will be helped.
DBS isn’t for everyone
As with the current DBS systems, patients must be carefully evaluated to see if they would benefit. As a rule, DBS is only suggested to patients if medication is failing to control symptoms or is causing serious side effects.
While DBS often works well in controlling many of the symptoms of Parkinson’s, it doesn’t work for everyone. The new device is expected to help no more than ten percent of the Parkinson’s population—an estimated one million people in the United States alone. Dr. Whone elaborates, saying, “If you’re more elderly, or if you’ve got memory problems as part of your Parkinson’s, this wouldn’t be suitable for you. But if you’re a younger person with Parkinson’s, who can have brain surgery and so on, then it becomes much more applicable to that group.”